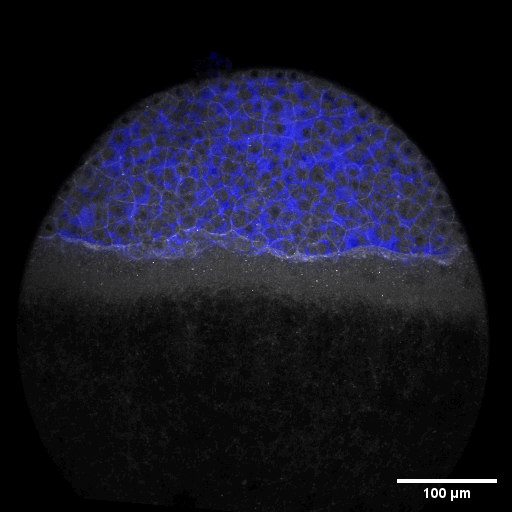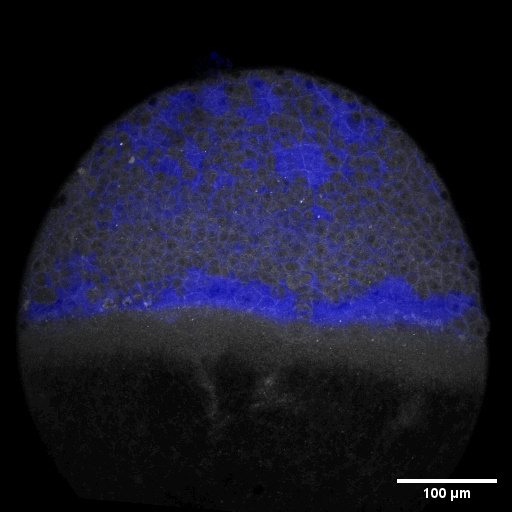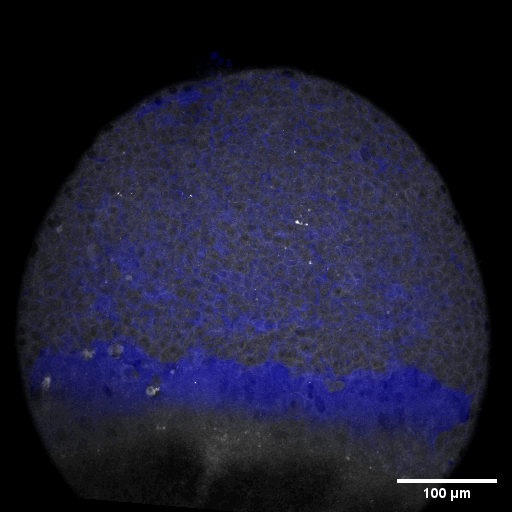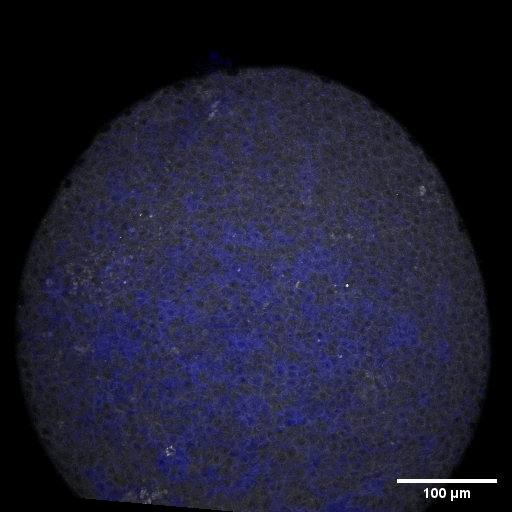
Cell adhesion, polarisation, movement and division stand in the center of our interest. As we aim to understand their role in shaping the embryo, a wide variety of molecular, cellular, and biophysical approaches is used. This allows us to unravel how mechanical forces are generated, transduced, received and sensed within the developing embryo, which ultimately determines how the embryo takes its shape.
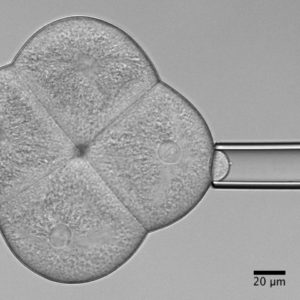 Zebrafish and ascidian embryos were chosen as model organisms to study tissue, organ and embryo morphogenesis, as they are transparently ex-utero developing chordates, which are easily accessible for experimental manipulations and live imaging. The unique embryonic structures of both model systems allow for the investigation of not only the fundamental morphogenetic mechanisms but also the evolutionary divergence and similarities.
Zebrafish and ascidian embryos were chosen as model organisms to study tissue, organ and embryo morphogenesis, as they are transparently ex-utero developing chordates, which are easily accessible for experimental manipulations and live imaging. The unique embryonic structures of both model systems allow for the investigation of not only the fundamental morphogenetic mechanisms but also the evolutionary divergence and similarities.
Ascidian embryos display an invariant cell lineage, allowing us to directly investigate the relationship between cell/tissue morphogenesis and cell fate specification. Drosophila has recently been incorporated as another model organism to explore cellular syncytium’s mechanical properties throughout embryogenesis.
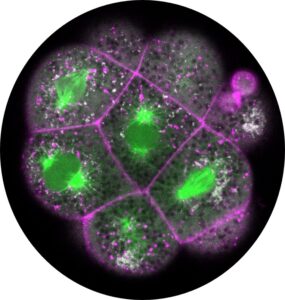
Interested in the crosstalk between biomechanical and biochemical mechanisms in the developing embryo, we have recently also started exploring the external and internal effects on biochemical signals, specifically cellular metabolism’s interaction with embryonic morphology. This includes new omics-analysis combined with novel imaging techniques to detect the direct physical interaction of metabolism with the dynamic embryonic cytoskeleton.